Revolutionizing subcutaneous administration of antibodies
RecoGel polymeric platform can convert any antibody intravenous (IV) formulation into a formulation for subcutaneous administration. RecoGel is a polysaccharide-based hydrogel, that can be co-formulated with antibodies at high concentration. After subcutaneous injection, RecoGel, loaded with the antibodies, will form a hydrogel in physiological conditions, allowing the controlled release of antibodies.
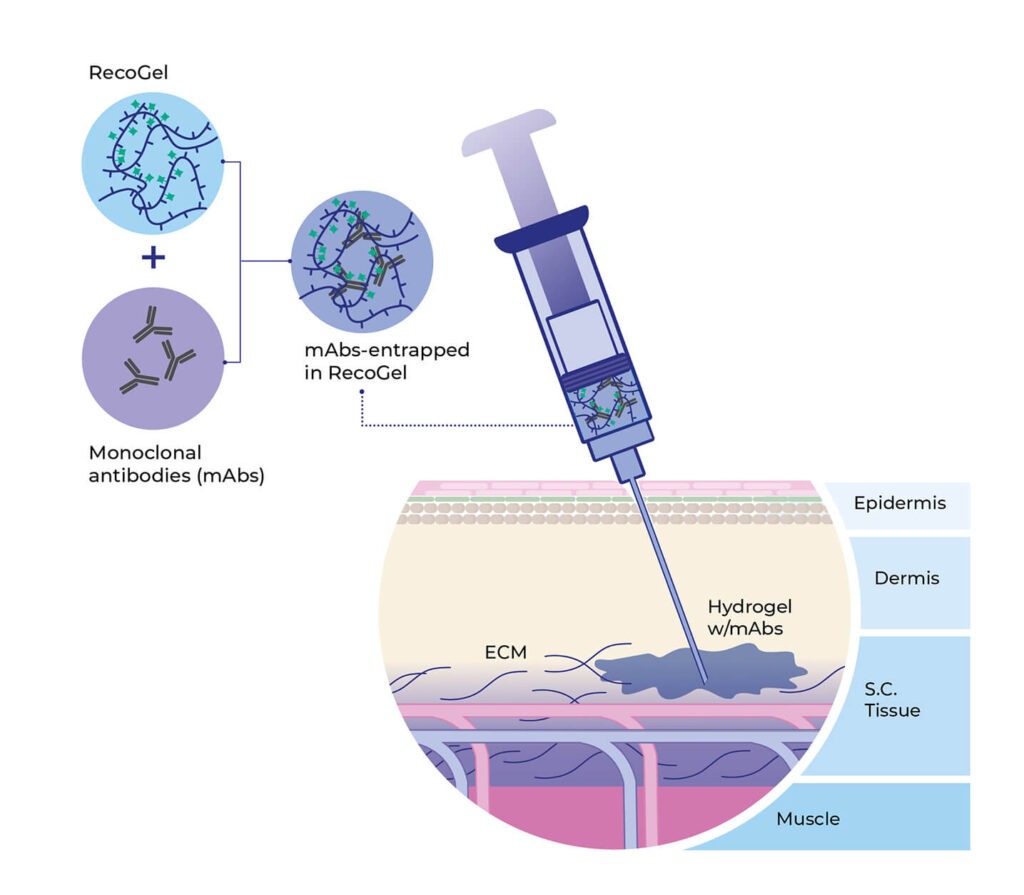
An unique hydrogel platform for subcutaneous delivery of antibodies
Simple conversion from IV to SC
High dose, up to 200 mg/mL (almost 2x greater concentration level versus standard SC formulation)
Compatibility across various antibodies

Biocompatible formulation

Biodegradable hydrogel
Controlled and tunable release of the antibody

PATIENTS
Better quality of life and mobility

HEALTHCARE PROFESSIONALS
Time savings and better use of dedicated staff

HOSPITALS/PAYERS
Reduced medical resource utilization and treatment costs

PHARMACEUTICAL INDUSTRY
Competitive advantage with a cutting edge delivery platform
Benefits address the full value chain
Many biological drugs are currently administered via intravenous infusion, which are burdensome for both patients and healthcare professionals.
Recobia Therapeutics wants to offer a better alternative to patients thanks to subcutaneous formulations and intends to alleviate their treatment burden with a faster and less painful administration.
Expanding access to subcutaneous formulations would also streamline healthcare professionals’ time and lower treatment costs, thereby improving treatment accessibility for a larger population.
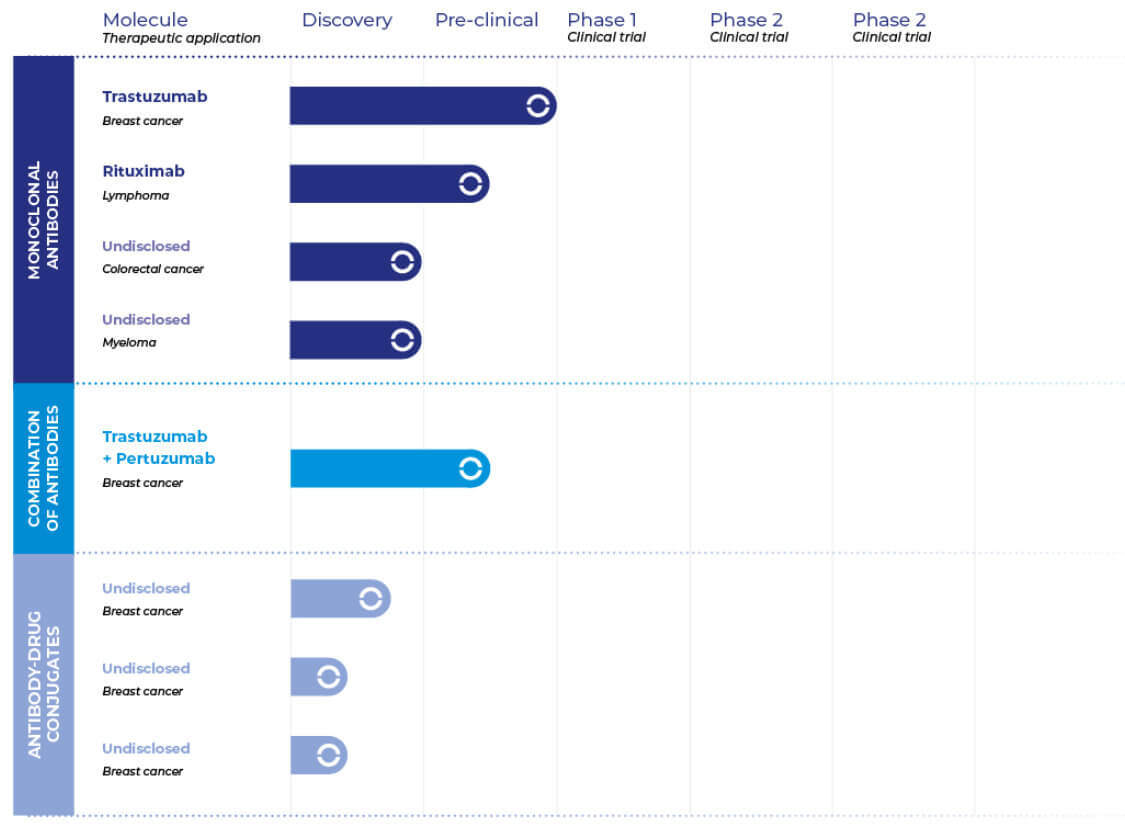
Discover our pipeline
| Molecule Therapeutic application |
Discovery | Pre-clinical | Clinical Studies | ||
|---|---|---|---|---|---|
Monoclonal Antibodies (mAbs) |
Trastuzumab Breast cancer |
||||
| Pembrolizumab Multiple indications |
|||||
| Rituximab Lymphoma |
|||||
Antibody-drug conjugates (ADC) |
T-DM1 Breast cancer |
||||
Bi-specific antibodies |
Undisclosed | ||||
Peptides |
Undisclosed |